Acidity of Alcohols/Ethanol, Why is phenol more acidic than other alcohols
Phenol Reaction
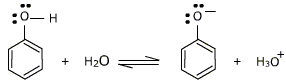
Acidity of phenol in reference to general alcohols is a common topic people have to address while studying about alcohols. I will attempt to provide a general background and simple explanation.
An acidic substance is something that can produce a hydrogen ion (H+), or H3O+ when in water. Few examples of common acids are HCl, H2SO4.
An alcohol is a chemical substance with a general formula of CnH2n+1OH. -OH is the functional group known as hydroxyl and that is what makes a chemical an alcohol. Some common alcohols are ethanol (C2H5OH), Methanol (CH3OH).
Ok, Now we are clear what an acidic substance and an alcohol is, lets move into the main topic of acidity of alcohol and phenol in particular.
In general, general alcohols and phenols, i.e compounds that contain the hydroxyl( -OH) group attached to a hydrocarbon are very weak acids. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can, and are usually virtually ignored.
This is usually the case as it is very tough to remove the H ion from the alcohol. I will not get into the specifics of why that is though, but it is mainly due to the stability of the compounds in different states, and the bond between Oxygen and Hydrogen.
However, the cyclic alcohol ,phenol has a sufficient acidic properties . It is however a very very weak acid when compared to other acids but more acidic when compared to other alcohols like ethanol. A hydrogen ion can break away from the -OH group and transfer to a base.
Ok, So you must be thinking now that i just said above that its difficult to remove the Hydrogen ion (H+) from alcohols due to stability issues, and the oxygen hydrogen bond.
Note: In general any chemical compounds prefer not to stay in a charged state.
So, lets try to understand why phenol can lose that hydrogen ion better than other alcohols. Phenol can lose a hydrogen ion because the phenoxide ion formed is stabilised to some extent. This is as the negative charge on the oxygen atom is delocalised around the ring, i.e having a cyclic structure in phenol helps it stay stable even after the hydrogen is removed. De-localized charge means, that the negative charge is not directly against any single point on the compound but the charge is distributed almost equally through the compound. The more stable the ion is, the more likely it is to form. In this case of phenol, the alcohol is almost similar to being non-charged even after the hydrogen ion is removed and thus can have some acidic effect.